Cell:Neuronal Small RNAs Control Behavior Transgenerationally
Highlights
- •C. elegans neuronal small RNAs are characterized by RNA sequencing
- •RDE-4-dependent neuronal endogenous small RNAs communicate with the germline
- •Germline HRDE-1 mediates transgenerational regulation by neuronal small RNAs
- •Neuronal small RNAs regulate germline genes to control behavior transgenerationally
Summary
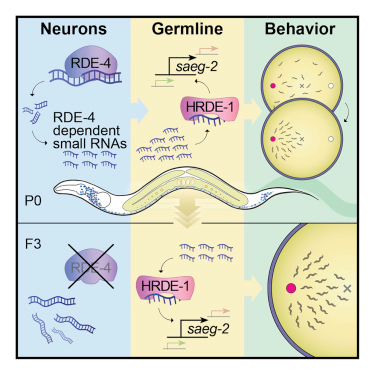
It is unknown whether the activity of the nervous system can be inherited. In Caenorhabditis elegans nematodes, parental responses can transmit heritable small RNAs that regulate gene expression transgenerationally. In this study, we show that a neuronal process can impact the next generations. Neurons-specific synthesis of RDE-4-dependent small RNAs regulates germline amplified endogenous small interfering RNAs (siRNAs) and germline gene expression for multiple generations. Further, the production of small RNAs in neurons controls the chemotaxis behavior of the progeny for at least three generations via the germline Argonaute HRDE-1. Among the targets of these small RNAs, we identified the conserved gene saeg-2, which is transgenerationally downregulated in the germline. Silencing of saeg-2 following neuronal small RNA biogenesis is required for chemotaxis under stress. Thus, we propose a small-RNA-based mechanism for communication of neuronal processes transgenerationally.
Graphical Abstract
Keywords
- transgenerational inheritance
- epigenetic inheritance
- small RNA inheritance
- non-Mendelian inheritance
- neuronal small RNAs
- C. elegans
Introduction
Among the different tissues of the body, the nervous system’s ability to collect and organize information about the environment is unmatched. Neuronal mechanisms of perception evolved to sense and interpret external and internal states and to orchestrate adaptive physiological responses fittingly. Ever since antiquity (Stubbe, 1972), many have speculated that brain activity could somehow generate heritable changes that would impact the fate of the next generations. The possibility that the nervous system can control the progeny could have far-reaching consequences.This idea, however, despite its appeal, challenges one of the basic dogmas of biology, also known as “the second law of biology” (Mattick, 2012). The “Weismann Barrier” (Weismann, 1891) asserts that the heritable information in the germline is segregated from somatic influences. Accordingly, animals’ responses to environmental challenges should not become inherited. More specifically, if the “Weismann Barrier” is indeed impermeable, then the consequences of neuronal activity should never affect the progeny. Nevertheless, a number of studies suggested that neuronal responses in parents can affect the offspring’s behavior. While these examples are fascinating, the exact underling mechanisms remain unknown (Weaver et al., 2004, Remy, 2010, Vassoler et al., 2013, Dias and Ressler, 2014, Gapp et al., 2014a).In the nematode Caenorhabditis elegans, small interfering RNAs that derive from artificial, exogenously supplied double-stranded RNA (exo-small interfering RNAs [siRNAs]) move from somatic cells, including neurons, to the germline (Fire et al., 1998, Devanapally et al., 2015). Further, an elaborate dedicated regulatory pathway has evolved to control transgenerational transmission of small RNA-initiated RNAi (Alcazar et al., 2008, Houri-Ze’evi et al., 2016, Houri-Zeevi and Rechavi, 2017, Lev et al., 2017, Spracklin et al., 2017). Transgenerational gene regulation depends on the amplification, by RNA-dependent RNA polymerases (RdRPs), of heritable small RNAs that bind specialized Argonautes in the germline, such as HRDE-1 (heritable RNAi deficient 1) (Aoki et al., 2007, Buckley et al., 2012). Environmental challenges (e.g., starvation and high temperatures) modulate the pool of heritable small RNAs and produce responses that last for multiple generations (Rechavi et al., 2014, Anava et al., 2015, Ni et al., 2016).Like many other organisms, nematodes naturally produce, in the soma and in the germline, endogenous siRNAs (endo-siRNAs) that align to multiple loci across the genome. Endo-siRNAs target both protein-coding and non-protein coding loci (Gu et al., 2009, Vasale et al., 2010). Endo-siRNAs align in the antisense orientation to exons, can tile the entire length of the mature mRNA transcript, and complement the target perfectly (Blumenfeld and Jose, 2016). For simplicity, units of small RNAs targeting a specific gene, will be referred to here as STGs (see Rechavi et al. 2014) (see STAR Methods).We hypothesized that biogenesis of neuronal endo-siRNAs could produce a heritable response. Endo-siRNAs were shown to control several neuronal functions affecting behavior and learning (Juang et al., 2013, Sims et al., 2016, Tonkin and Bass, 2003) and to mediate transgenerational gene regulation in the germline (Ashe et al., 2012, Shirayama et al., 2012, Rechavi and Lev, 2017). We focused on neuronal endo-siRNAs that depend on the double-stranded RNA (dsRNA)-binding protein RDE-4 (RNAi deficient 4). RDE-4 acts upstream in a biogenesis pathway that generates endo-siRNAs (Vasale et al., 2010, Welker et al., 2010, Lee et al., 2006, Duchaine et al., 2006, Gu et al., 2009) and is important for several neuronal functions, including migration of the HSN neuron, learning, and memory (Tonkin and Bass, 2003, Kennedy and Grishok, 2014, Juang et al., 2013).To study the heritable effects of neuronal small RNAs, we engineered multiple transgenic strains in which we rescued RDE-4’s expression specifically in neurons of rde-4(−/−) worms. We found that RDE-4 controls the levels of various endo-siRNAs in neurons, but also, more intriguingly, in the germline. The function of the neuronal RDE-4-dependent germline endo-siRNAs depends on the germline-specific Argonaute HRDE-1 and regulates the expression levels of complementary mRNAs transgenerationally. Furthermore, we discovered that biogenesis of neuronal endo-siRNAs controls transgenerationally the capacity of worms to perform chemotaxis. We found that the conserved gene saeg-2 is regulated in the germline by neuronal RDE-4 in an HRDE-1-dependent manner across multiple generations, and saeg-2 silencing is key for proper chemotaxis. We propose that small RNA regulation is a mechanism that allows the nervous system to communicate with the germline affecting the behavior of the next generations.
you can click original link view full atricle: https://www.cell.com/cell/fulltext/S0092-8674(19)30448-9#articleInformation